Secukinumab 'significantly' more effective in DMARD-naïve patients with PsA
Release time:2021-02-08 01:19:00
Secukinumab is effective in psoriatic arthritis at 6 and 12 months, with significantly better remission, low disease activity, retention and response rates in patients naïve to disease-modifying antirheumatic drugs, according to data.
“The treatment options for PsA have improved during the last few decades with the introduction of biologic DMARDs and targeted synthetic DMARDs,” Brigitte Michelsen, MD, of Rigshospitalet Glostrup, in Denmark, and colleagues wrote in Arthritis Care & Research. “Nevertheless, a recent real-world study of >14,000 patients with PsA, who started treatment with a TNF inhibitor, showed that less than half of the patients had achieved clinical remission after 6 months. Thus, there is an unmet need for other treatment options in patients with PsA.”
Secukinumab is effective in PsA at 6 and 12 months, with significantly better remission, low disease activity, retention and response rates in patients who are naïve to biologic/targeted synthetic DMARDs, according to data. Source: Adobe Stock
“The fully human IgG monoclonal IL-17A inhibitor secukinumab was approved for use in PsA patients in the European Union in 2015,” they added. “Secukinumab has demonstrated good efficacy and safety in randomized controlled trials, whereas large observational studies on its effectiveness in patients with PsA are lacking.”
To examine the real-life effectiveness — including retention, remission, low disease activity and response rates — of secukinumab (Cosentyx, Novartis) among patients with PsA at 6 and 12 months, Michelsen and colleagues analyzed data from 13 countries in the European Spondyloarthritis Research Collaboration Network. Registries included in their analysis were ARTIS, of Sweden; DANBIO, from Denmark; SCQM, from Switzerland; GISEA, of Italy; BIOBADASER, from Spain; ATTRA, from the Czech Republic; Biorx.si, of Slovenia; Reuma.pt, from Portugal; NOR-DMARD, from Norway; ROB-FIN, of Finland; ICEBIO, from Iceland; RRBR, of Romania; and TURKBIO from Turkey.
For their study, Michelsen and colleagues analyzed data from 2,017 adults with PsA who initiated secukinumab for the first time between May 2015 and December 2018. The registries provided longitudinal, observational data collected as part of routine care.
Data included age, gender, time since diagnosis, current smoking status, BMI, secukinumab start and stop dates, previous DMARD treatment, evaluator’s global assessment, patient’s global assessment, pain and fatigue, C-reactive protein (CRP, mg/L), erythrocyte sedimentation rate (ESR, mm/h), 28-joint Disease Activity index for Psoriatic Arthritis (DAPSA28), 28 joint Disease Activity Score with CRP (DAS28-CRP), Clinical Disease Activity Index (CDAI)15 and the Simplified Disease Activity Index (SDAI).
The primary outcome was the overall 12-month secukinumab retention rate. Secondary outcomes included the overall 6-month secukinumab retention rate and 6- and 12-month remission, as well as low disease activity and response rates.
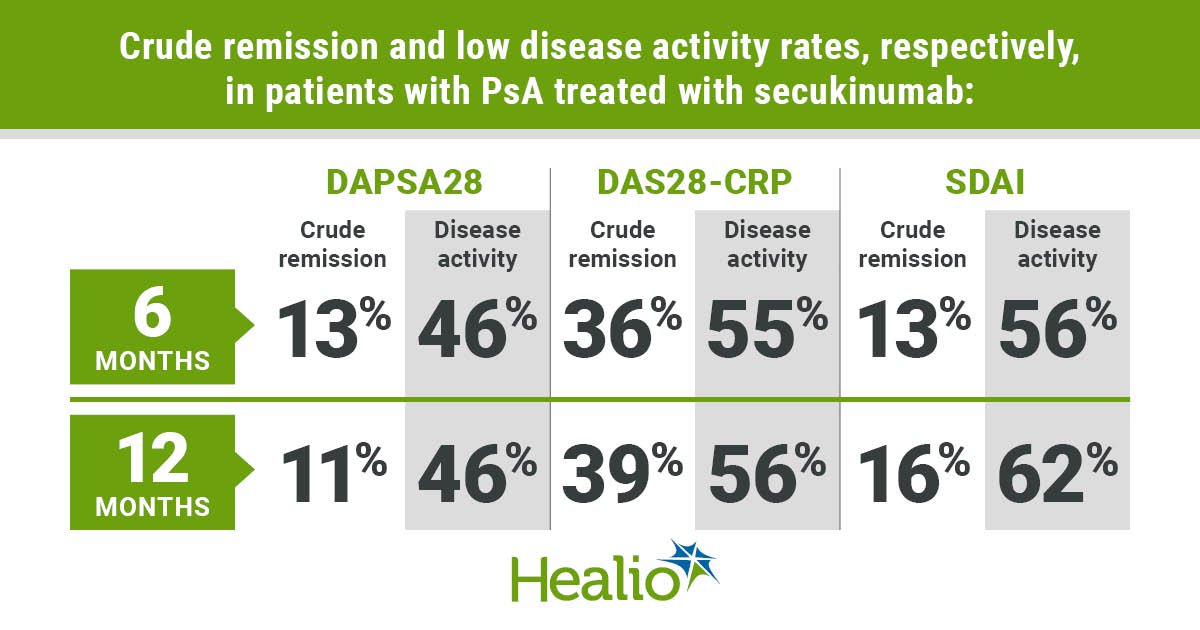
According to the researchers, overall retention rates with secukinumab were 86% after 6 months and 76% after 12 months. Crude — LUNDEX adjusted — remission and low disease activity rates at 6 months were 13% and 46%, respectively for DAPSA28, 36% and 55% for DAS28‐CRP, and 13% and 56% for SDAI. At 12 months, crude remission and low disease activity rates were 11% and 46%, respectively for DAPSA28, 39% and 56% for DAS28‐CRP and 16% and 62% for SDAI. CDAI remission and low disease activity rates were similar to the SDAI rates. ACR20/50/70 responses were 34%, 19% and 11%, respectively, at 6 months, and 37%, 21% and 11% at 12 months.
“Overall, high 6-month (86%) and 12-month (76%) secukinumab retention rates were found,” Michelsen and colleagues wrote. “Secukinumab effectiveness was significantly better for bio-naïve patients after 6 as well as 12 months of treatment, was independent of time since diagnosis and differed significantly across the European countries. Remission, LDA and response rates were overall comparable to previous real-life observations in patients treated with a TNFi. Hence, this large observational study documents the effectiveness of secukinumab in the treatment of PsA patients.”
References:
https://www.healio.com/news/rheumatology/20210203/secukinumab-significantly-more-effective-in-dmardnave-patients-with-psa